Benefits of PIC/S GMP membership
On January 1st, 2021, ANVISA from Brazil became the 54th member of PICS. Like other members of PICS, both ANVISA and Brazil pharma industry will realize the huge benefits of PIC/S membership. What are the benefits of becoming a PIC/S member?
On January 1st, 2021, ANVISA from Brazil became the 54th member of PICS. Like other members of PICS, both ANVISA and Brazil pharma industry will realize the huge benefits of PIC/S membership.
What are the benefits of becoming a PIC/S member? This article will address the issue. Let’s get into it!
1. Members of PICS
Over the past 20 years, the number of participating authorities increased from 23 to 54. Currently, there are 5 registered and 3 pre-registered to prepare to become a member of PICS.
It usually takes 3 - 4 years for applicants to become PIC/S members. The pre-application process provides the regulator with the opportunity to analyze the loopholes and determine whether they are ready to submit a full application.
Read more: What is PIC/S GMP?

2. Benefits for members of PICS
To become a PICS member, applicants must make many improvements to the policy, inspection procedures, quality system, training, etc to catch up with the existing members' systems and procedures. The PICS membership process requires applicants to develop the system and procedure for international GMP harmonization. When becoming a member, these standards need to be maintained.
Moreover, there are many other benefits, like:
Sharing GMP inspection reports among authorities is carried out. This is relevant at this time of Covid-19 pandemic when Regulatory Authorities are unable to travel to conduct on-site foreign inspection
Information sharing is a cost-saving measure because the trips for inspection have reduced considerably. Currently, that PICS authorities carry out remote inspections is common in the Covid-19 pandemic.
Involving in GMP inspection practices
“PICS remote inspection” guideline document is becoming more and more important in Covid-19 pandemic.
In December 2020, PICS also organized a Virtual training seminar for GMP inspectors on “ Remote assessment of GMP compliance”. The consequence of the seminar is that a group was established to prepare for guideline documentation related to the topic of the seminar.
GMP inspector training opportunities: New inspector training, annual PICS seminar on a particular topic, etc
3. Benefits for pharmaceutical industry
When the Authority becomes a member of PIC/S, the pharmaceutical industry also gains benefits from PIC/S GMP. One of the benefits is pharmaceutical export facilitation for both PICS member countries and non-PICS countries.
When becoming a PICS member in 1995, Australia experienced considerable growth in the export of pharmaceuticals in the following years.
Growth in pharmaceuticals export of Australia due to membership in PIC/S is presented as below:
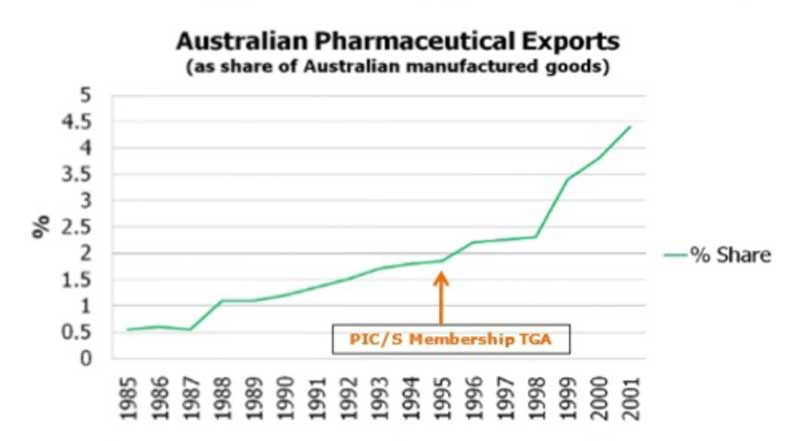
Image: Pharmaceutical export growth in Australia accelerated by membership PICS
Other benefits for the pharma industry are reduced duplication of GMP inspection of the same region and cost-savings, especially during Covid-19 pandemic when PIC/S authorities are forced to rely more on sharing inspection reports and other information related to GMP inspection. It is possible that this approach will continue after the pandemic is over, although not yet finalized.
Before Covid-19 pandemic, many pharma companies had undergone multiple GMP inspections of the same region carried out by different regulatory authorities, which were a significant cost burden for the industry. According to the report, inspected companies need 10 times more resources than regulatory authorities to prepare and conduct PICS GMP inspections.
Read more: What're the differences between WHO GMP and PIC/S GMP?