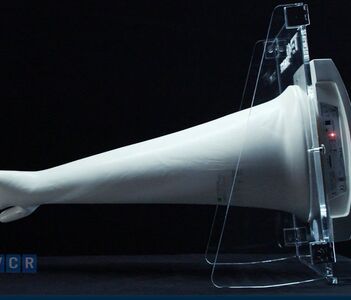
How to check the quality of CAM gloves: CO, CQ, durability, chemical resistance
How to test the quality of CSM gloves is a key factor to ensure chemical safety and compliance in the pharmaceutical, chemical and cleanroom industries. With strong chemical resistance, high mechanical strength and outstanding ozone - UV resistance, CSM gloves need to be evaluated by CQ, CO, durability and chemical resistance.
Read More