Cost of periodic maintenance of pharmaceutical clean room air filtration system
The cost of regular maintenance of pharmaceutical cleanroom air filtration systems is not only an investment to maintain stable operation but also a mandatory requirement according to GMP standards. Proper periodic maintenance helps prevent cross-contamination, prolongs equipment life and ensures safe drug quality.
- 1. Why is Regular Maintenance of Air Filtration Systems Necessary in Pharmaceutical Cleanrooms?
- 2. Key Maintenance Tasks and Recommended Frequency
- 3. What Does Cleanroom Air System Maintenance Cost Include?
- 4. Factors Affecting Cleanroom Air System Maintenance Costs
- 5. How to Optimize Maintenance Budgets While Meeting GMP Standards
- 6. Frequently Asked Questions About Cleanroom Air System Maintenance
- 7. Need a Maintenance Cost Estimate for Your Pharmaceutical Cleanroom?
1. Why is Regular Maintenance of Air Filtration Systems Necessary in Pharmaceutical Cleanrooms?
The air filtration system is the "lungs" of a pharmaceutical cleanroom, ensuring cleanliness levels are maintained according to GMP standards (EU-GMP, WHO-GMP). Regular maintenance not only helps the equipment operate stably but is also a mandatory requirement during audits and quality inspections by regulatory authorities.
Strict Compliance with GMP Standards
Regulations such as EU-GMP Annex 1 require pharmaceutical facilities to have a clear maintenance plan for the entire HVAC system, FFUs, HEPA boxes, and air control equipment. Tasks like filter replacement, DOP testing, and pressure monitoring must follow a scheduled plan with full documentation to support audits.
Failing to maintain the system regularly can lead to the cleanroom losing its GMP certification, resulting in legal and financial consequences.
Preventing Cross-Contamination and Production Risks
When filters are overloaded or clogged, airflow in the cleanroom becomes disrupted, leading to:
- Loss of positive pressure → contaminated air backflow
- Ineffective removal of dust and microorganisms
- Cross-contamination between areas (especially in weighing or filling zones)
This directly impacts product quality and can lead to product recalls or production suspension.

Extending Equipment Lifespan and Reducing Long-Term Costs
Regular maintenance allows equipment like FFUs, HEPA boxes, and AHUs to run smoothly and helps avoid unexpected failures. Specifically:
- Timely pre-filter replacement protects downstream HEPA filters
- FFU motor checks help detect issues early
- Regular pressure monitoring warns of clogged filters
The cost of periodic maintenance is significantly lower than the cost of full system replacement or production downtime due to malfunctions.
2. Key Maintenance Tasks and Recommended Frequency
Establishing a routine maintenance schedule for cleanroom air systems is critical to GMP compliance and proactive risk management. Below are essential maintenance tasks and suggested frequencies based on operational experience in pharmaceutical facilities:
|
Maintenance Task |
Recommended Frequency |
Details |
|
Pre-filter Replacement |
Every 1-3 months |
Depends on ambient air quality; regular checks help prevent clogging. |
|
FFU / AHU Cleaning |
Every 3-6 months |
Check fan motors, clean blades, and measure airflow for optimal output. |
|
Pressure Differential Monitoring |
Monthly |
Use differential pressure gauges to monitor airflow integrity. |
|
HEPA Filter Replacement |
Every 12-24 months |
Based on DOP test results; clogged HEPA filters reduce airflow quality. |
|
DOP Testing (HEPA integrity check) |
Every 6-12 months |
Mandatory under EU-GMP Annex 1 to ensure microbial filtration efficiency. |
Note: For critical areas such as weighing, filling, or packing rooms (ISO 7-8), maintenance frequency should be increased to detect issues early.
See more: What is an AHU? Structure and operating principles of the AHU system
3. What Does Cleanroom Air System Maintenance Cost Include?
When planning for air system maintenance in pharmaceutical cleanrooms, it’s important to consider all associated costs-not just materials, but also labor, testing, and potential downtime. Here's a breakdown of typical costs to budget for:
Consumable Materials
- Pre-filters: Replaced every 1-3 months, typically VND 300,000-500,000 per unit
- HEPA filters: Replaced every 12-24 months, VND 3,000,000-5,000,000 per unit (depending on size and efficiency)
- Accessories: Gaskets, filter frames, support structures as needed
Labor Costs
- Includes inspection, disassembly, cleaning, and FFU/AHU adjustments
- Average cost: VND 2,000,000-5,000,000 per room, depending on system complexity
Testing & Calibration
- DOP testing for HEPA filters: Mandatory under GMP
- Pressure and airflow measurement: Ensures system performance and compliance
- Pricing typically depends on the number of devices or room area
Production Downtime (if applicable)
- Maintenance during operating hours may disrupt production schedules
- Solution: Schedule during off-hours or opt for “express maintenance” services
Sample Cost Table (per maintenance item)
|
Item |
Estimated Cost (VND) |
Notes |
|
Pre-filter Replacement |
300,000 - 500,000 per unit |
Depends on type and application area |
|
HEPA Filter Replacement |
3,000,000 - 5,000,000 per unit |
Higher for premium filters |
|
Labor (Full Room Service) |
2,000,000 - 5,000,000 per room |
Includes inspection, cleaning, and reporting |
|
DOP Testing |
Based on scope (contact for quote) |
Required for GMP compliance |
Note: These are average market prices. Actual costs may vary depending on facility size, number of units, and ISO classification requirements.
4. Factors Affecting Cleanroom Air System Maintenance Costs
The cost of maintaining air filtration systems is not fixed; it varies significantly depending on the technical configuration and level of investment of each facility. Here are four key factors that determine actual maintenance costs:
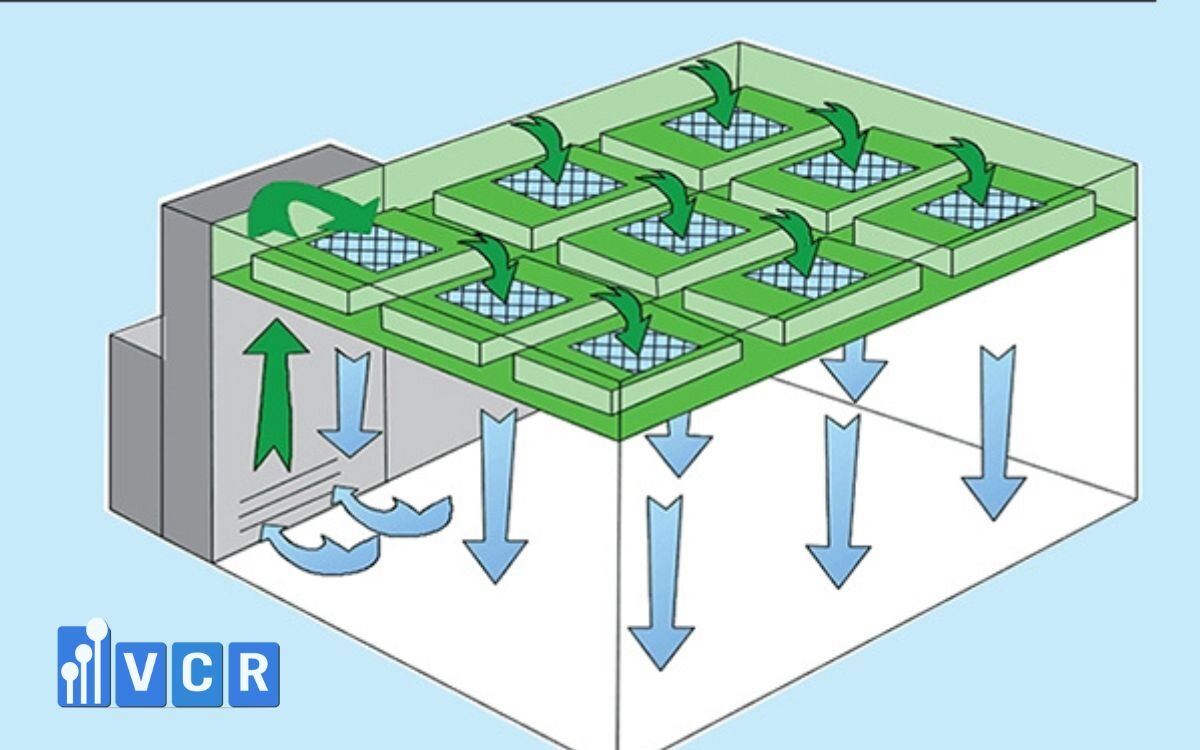
Number of FFUs and HEPA Boxes in the System
- The more equipment installed, the higher the workload for maintenance, and therefore, the cost increases accordingly.
- Each FFU must be checked for motor condition, airflow measurement, mesh cleaning, and potential filter replacement.
- HEPA boxes require DOP testing and filter replacement if standards are not met.
Example: A cleanroom with 50 FFUs will cost 2-3 times more to maintain than a room with only 20 FFUs.
Pollution Level in the AHU Intake Area
- If the AHU draws air from an environment with high dust, fumes, or humidity, filters will clog faster.
- Pre-filters must be replaced more frequently, increasing material and labor costs.
Solution: Install a coarse dust filter screen outside the AHU to reduce the load on the pre-filter.
Cleanroom Size and ISO Classification (ISO 5 to ISO 8)
- The lower the ISO classification (the cleaner the area), the stricter the maintenance requirements.
Examples:
- ISO 5 room → DOP testing every 6 months, high-performance HEPA filters
- ISO 8 room → DOP testing every 12 months, simpler filtration system
Filling or packaging rooms in the pharmaceutical industry typically require ISO 7-8 standards, so separate maintenance budgeting is necessary.
Use of Pass Boxes and Air Showers
- These auxiliary devices also contain filters that require maintenance.
- Air showers must be cleaned, and the nozzles, pressure levels, and pre-filters must be inspected.
- Pass boxes (especially electronic types) require fan, sensor, and interlock checks.
If a pharmaceutical facility uses multiple pass boxes or air showers, overall maintenance costs may increase by 10-20%.
See more: Comparison between HEPA H13 Filter and HEPA H14 Filter
5. How to Optimize Maintenance Budgets While Meeting GMP Standards
Reducing costs does not mean compromising on quality. In the pharmaceutical industry, it is crucial to optimize budgets while fully complying with GMP requirements. Here are four practical ways to control costs without compromising cleanroom air system safety:
1. Use Certified Filters with Longer Lifespan
- Choose HEPA and pre-filters from reputable brands with certified filtration efficiency ≥99.97% (for HEPA).
- Although the initial price is higher, their lifespan is 20-30% longer than low-cost alternatives.
- This reduces replacement frequency, saving both material and labor costs.
Investing in high-quality filters can reduce at least 1-2 maintenance sessions per room annually.

2. Sign Quarterly or Annual Maintenance Contracts
- Instead of booking separate maintenance sessions, sign long-term contracts with cleanroom service providers.
Benefits:
- 10-20% discount on total cost
- Ensures timely maintenance scheduling without oversight
- Regular reports available for GMP audits
VCR offers annual maintenance packages for pharmaceutical plants, including pressure measurement, HEPA testing, and pre-filter replacement.
3. Use Remote Monitoring Dashboards (If Available)
- For large systems, consider a dashboard connected to sensors that monitor pressure, temperature, and airflow.
- When anomalies occur (e.g., pressure drops or filter blockages), the system provides early alerts.
- This allows detection before major damage occurs, reducing repair costs.
Some modern AHU/FFU models include built-in monitoring or allow sensor module integration.
4. Create Checklists and Train In-House Maintenance Staff
- Many maintenance issues stem from improper handling or incomplete inspections.
Solution:
- Develop standardized GMP maintenance checklists
- Train internal staff to read pressure gauges, inspect filters, and clean FFUs properly
VCR provides sample maintenance checklists for air filtration systems in compliance with EU-GMP standards - available upon request.
6. Frequently Asked Questions About Cleanroom Air System Maintenance
1. Is it mandatory to replace HEPA filters regularly, or only when they fail?
→ Yes. According to GMP standards (EU, WHO), HEPA filters must be replaced every 12-24 months or immediately if they fail a DOP test. Waiting until failure can lead to contamination and loss of environmental control effectiveness.
2. Do ISO 8 cleanrooms require monthly pressure differential checks?
→ Yes. Whether ISO 5 or ISO 8, maintaining stable positive pressure is essential to prevent contaminated air ingress. GMP requires regular pressure monitoring using differential pressure gauges and documented records for inspections.
3. Do FFUs require maintenance, or is filtration alone sufficient?
→ Maintenance is necessary. Each FFU (Fan Filter Unit) contains a fan, motor, and HEPA filter. It should be regularly checked for:
- Airflow speed
- Vibration and noise levels
- Motor performance
Without proper maintenance, FFUs can lose efficiency or disrupt room pressure balance.
4. What is the typical annual maintenance cost for a pharmaceutical cleanroom?
→ Costs range from VND 20-50 million per room per year, depending on:
- Room size
- Number of FFUs/HEPA boxes
- ISO classification (ISO 5, 7, 8)
- Frequency of DOP testing and pressure checks
This estimate covers the air filtration system only. Other systems like HVAC, interlocks, or pass boxes will incur additional costs.
7. Need a Maintenance Cost Estimate for Your Pharmaceutical Cleanroom?
Proper GMP-compliant maintenance isn’t just a requirement - it’s essential protection for your pharmaceutical production line.
VCR offers comprehensive air system maintenance packages for pharmaceutical facilities:
- Replacement of certified HEPA and pre-filters
- Regular pressure, airflow, and DOP testing
- Complete documentation for GMP compliance
- Cost optimization tailored to room size and ISO class
Contact us today for consultation and a free quotation:
Hotline: 090.123.9008
Email: [email protected]
Website: https://vietnamcleanroom.com/
Diep VCR














