How to test air flow in food factory
Airflow testing in a food factory is an important factor in preventing cross-contamination between production areas. Measuring and checking the air direction, speed and differential pressure helps ensure that the air flow always moves in the right direction, maintaining a clean environment that meets food safety and GMP standards.
- 1. Why is airflow validation necessary in food processing plants?
- 2. When should airflow validation be conducted?
- 3. Key parameters to measure during airflow validation
- 4. GMP-Compliant Airflow Validation Process
- 5. Common Airflow Validation Issues and Solutions
- 6. Recommended Equipment for Effective Airflow Validation
- 7. Frequently Asked Questions
- 8. Need HACCP/GMP-Compliant Airflow Validation?
In food processing plants, maintaining proper airflow not only helps control cleanliness but also ensures product quality and compliance with sanitation standards. So how to test air flow in food factory work in a food production environment - from determining flow rates, air speeds to pressure differentials between areas - and why is it becoming increasingly important? This article will guide you through the steps of airflow testing in a food plant, helping you to follow the right techniques and avoid operational errors.
1. Why is airflow validation necessary in food processing plants?
In food production environments, airflow is not just for ventilation-it plays a critical role in controlling microbiological and particulate contamination. If airflow is not properly validated and maintained, the risk of cross-contamination between processing zones increases significantly, directly impacting product quality and food safety.
Cross-contamination risk from uncontrolled airflow
Food factories are typically divided into multiple zones: raw material intake, preliminary processing, mixing, filling, packaging, etc. Each zone has a different level of cleanliness. If airflow is not well controlled, contaminated air from "dirty" areas can flow into "clean" areas, leading to product contamination.
Example: Airflow from the washing area blowing into the filling zone may carry moisture, microorganisms, and unprocessed particulates.
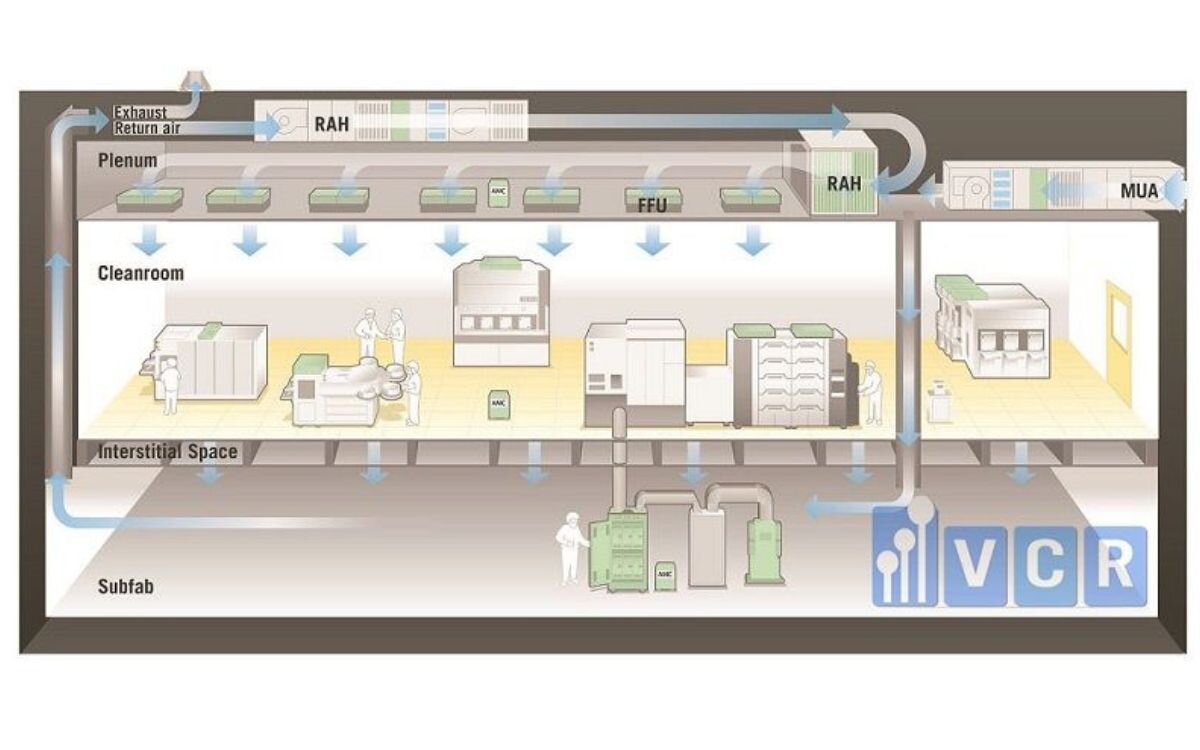
The role of airflow in controlling contaminants
The HVAC system in a food plant is responsible for more than just cooling. It ensures:
- Positive pressure in clean areas to prevent dust intrusion
- Filtration of bacteria, spores, and fine particles via HEPA filters
- Unidirectional airflow to maintain clear “clean-to-dirty” air movement
Validation helps confirm that all these elements are functioning as designed.
Compliance with food safety standards
International food safety standards require environmental control in production, and airflow is a mandatory component:
GMP (Good Manufacturing Practices): Requires proper airflow in clean areas to prevent contamination
HACCP (Hazard Analysis and Critical Control Points): Treats airflow as a critical control point (CCP) if contamination risk exists
ISO 22000: Requires evaluation and control of environmental factors affecting food safety, including air handling systems
Therefore, airflow validation is not just a technical recommendation-it is a requirement for achieving international quality certifications.
2. When should airflow validation be conducted?
Airflow validation in food plants should not be a reactive measure. Instead, it must be planned regularly or triggered by certain milestones to ensure the clean air system performs correctly and meets hygiene standards.
Before putting the plant into operation
Once construction, HVAC installation, FFU and HEPA filters, and functional zoning are complete, airflow validation should be conducted to:
- Verify airflow direction is correct (clean to dirty)
- Ensure pressure differentials meet required values
- Confirm that clean airflow is uninterrupted and laminar
This is a prerequisite for GMP audits or HACCP certification.
After modifications or HVAC maintenance
Every time the plant layout changes, new equipment is installed, or HVAC components are upgraded (e.g., HEPA filter replacement), airflow may be disrupted. Revalidation ensures:
- Early detection of abnormal airflow areas
- Prevention of temporary cross-contamination due to new layouts
- Assurance that the system still complies with design and hygiene standards

On a scheduled basis (every 6–12 months)
According to GMP and ISO 22000 guidelines, airflow validation must be conducted at least once a year. For high-risk operations (e.g., perishable foods, aseptic zones), validation should occur every 6 months.
Routine validation helps:
- Detect degradation in filtration or ventilation systems
- Evaluate performance of FFU, HEPA filters, Air Showers, etc.
- Comply with internal or third-party audits and inspections
See more: What is AHU? Structure and operating principle of AHU system
3. Key parameters to measure during airflow validation
Airflow validation cannot rely on visual observation alone. Specific technical measurements are essential to assess how effectively the air system controls the environment. The four critical parameters below must be checked during every validation:
Table of airflow validation parameters
|
Parameter |
Technical significance |
Recommended device |
|
Air velocity |
Measures the speed of clean air distribution. Stable and uniform velocity ensures effective airflow and prevents turbulence. |
Anemometer (Air velocity meter) |
|
Airflow direction |
Confirms whether the airflow follows the “clean to dirty” principle. Prevents reverse flow that may cause cross-contamination. |
Smoke Generator |
|
Pressure differential |
Measures pressure between zones. Positive pressure prevents contaminated air from entering clean areas. |
Differential pressure gauge (2-pointer or digital) |
|
Air volume (flow rate) |
Verifies the total airflow delivered to each area. Ensures HVAC/FFU meets design requirements. |
Flow Hood (Capture hood for air volume) |
Why are these parameters critical?
- Low air velocity → Particles are not effectively swept away → microbial buildup risk increases
- Reverse airflow → Microorganisms from dirty zones may enter clean zones
- Insufficient pressure differential → Clean air leaks or reverse flow occurs
- Low air volume → Inadequate airflow to maintain the required cleanroom class
Each parameter reflects the performance of a specific part of the clean air system. Accurate measurements and analysis enable timely corrections before any breach of control compromises product safety.
4. GMP-Compliant Airflow Validation Process
To ensure that the clean air system in a food processing plant functions effectively and meets standards, airflow validation must follow a standardized procedure in accordance with GMP guidelines and ISO 14644-1 standards.
Here are the five commonly applied validation steps:
Step 1: Prepare the validation area
Close all doors and windows; interlock systems must function properly
Turn off unrelated devices (local fans, unnecessary machinery)
Ensure the room is in a "ready for operation" state to guarantee valid measurement results
Proper preparation eliminates interference and ensures measurement accuracy.
Step 2: Conduct a Smoke Test to assess airflow direction
Use a smoke generator at transition points between rooms or near doors
Observe the smoke movement: it should flow from clean to dirty areas
Record video or snapshots for validation reports and GMP audits
Smoke tests provide a visual assessment of airflow direction, particularly critical in airlocks, gowning rooms, and filling areas.

Step 3: Measure airflow velocity using a standard grid
Use an anemometer to measure air velocity at fixed points on the ceiling (for FFUs) or in work zones
Points should be arranged in a 3x3 or 4x4 grid, depending on the area size
Compare readings with design specifications or ISO 14644-1 Class 7–8 requirements
The goal is to ensure uniform airflow with no dead zones or turbulence.
Step 4: Evaluate pressure differential between adjacent rooms
Measure pressure differentials using a 2-pointer or digital gauge
Check pressure between areas such as pre-processing vs. packaging, or airlock vs. corridor
GMP recommends a minimum differential of ≥10 Pa between clean and non-clean areas
Proper pressure differentials act as an invisible barrier preventing contamination.
Step 5: Compare results with international standards
Benchmark results against:
- ISO 14644-1 Class 7–8 (commonly used in food processing)
- GMP requirements from clients or authorities
- HACCP-defined environmental criteria
Document and compile a standard-format report for internal evaluation or external audits
See more: The differences between cleanroom HVAC system and other HVAC systems
5. Common Airflow Validation Issues and Solutions
Even with a correctly designed clean air system, technical issues may arise that compromise airflow balance. Below are common problems and suggested corrective actions:
Issue 1: Insufficient pressure differential between clean and general zones
Symptoms:
Pressure difference between production and hallway is <10 Pa
Backdraft sensation when opening doors
Causes:
- Weak FFU or exhaust fans
- Leaky doors or faulty interlock system
- Clogged HEPA filters
Solutions:
- Adjust supply/exhaust airflow
- Replace degraded HEPA filters
- Increase number or capacity of FFUs in clean zones
Issue 2: Reverse airflow at airlock doors
Symptoms:
Smoke test shows reverse flow from dirty to clean areas
Causes:
- Inadequate pressure between airlock compartments
- Both doors open simultaneously, no interlock system
- Poor fan placement causing trapped air
Solutions:
- Install interlock system to prevent simultaneous door opening
- Add 2-pointer pressure gauges at each airlock door
- Review airflow design into airlock zones
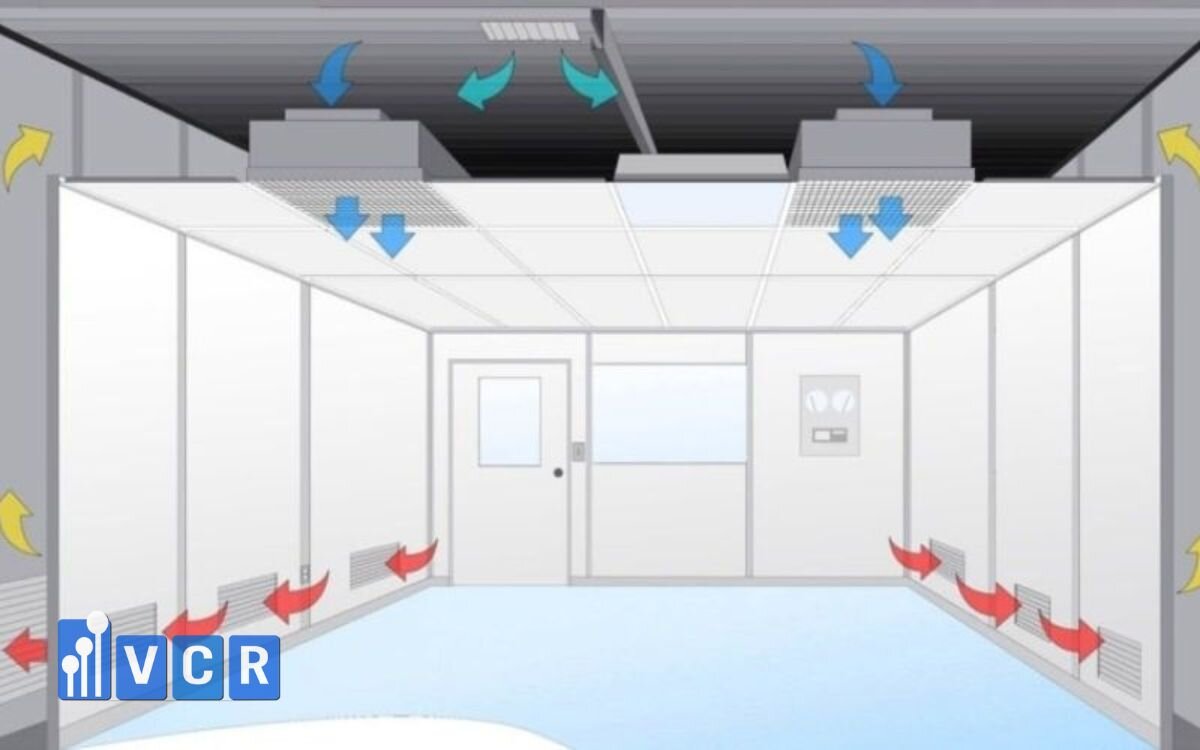
Issue 3: Uneven FFU performance
Symptoms:
Very low or no air velocity in some areas
Causes:
- Faulty FFU motors or inverters
- Dirty fan blades reducing performance
- Incorrect FFU installation (reverse airflow)
Solutions:
- Check FFU voltage and motor speed
- Clean pre-filters and internal fans regularly
- Re-align FFUs according to airflow schematics
Issue 4: Improper equipment layout obstructing airflow
Symptoms:
Turbulence or stagnant air zones in production areas
Increased particle deposition, harder to meet cleanliness class
Causes:
- Large machinery placed under supply vents
- Pallets or materials blocking return air vents
- Non-compliant layout design ignoring airflow principles
Solutions:
- Reassess room layout to maintain unidirectional flow
- Avoid placing obstructions under vents in clean zones
- Train staff on correct material and equipment positioning
See more: Air duct in cleanroom: Requirements and installation
6. Recommended Equipment for Effective Airflow Validation
Accurate and efficient airflow validation requires specialized equipment that complies with international standards. Recommended tools include:
ISO 14644-compliant FFUs
Provide unidirectional clean airflow directly from ceiling to work area
Maintain consistent airflow velocity and volume, simplifying validation
Two-pointer differential pressure gauges
Real-time monitoring with max-value recording
Easy installation in airlocks, process areas, and corridors
Smoke Generator / Airflow Test Kit
Visual tool for detecting airflow direction and reverse flow points
Widely accepted in GMP audits
Air velocity and volume meters (Anemometer + Flow Hood)
Flow Hood: measures total airflow at supply/return locations to verify uniform distribution
7. Frequently Asked Questions
1. Is routine airflow validation mandatory?
Yes. For food plants following HACCP, ISO 22000, or GMP systems, periodic airflow validation is mandatory to maintain a hygienic production environment and prevent cross-contamination.
2. Is smoke testing accurate for airflow direction?
Yes. Smoke tests are a simple, visual, and effective way to assess airflow direction. However, they should be combined with velocity measurements for reliable validation documentation.
3. How often should airflow systems be revalidated?
GMP and HACCP recommend revalidation at least every 12 months. Additionally, it should be conducted after significant changes to the HVAC system, room layout, or air-handling equipment.
8. Need HACCP/GMP-Compliant Airflow Validation?
Airflow validation is not just a technical requirement-it’s a sign of commitment to food safety for regulators and partners.
If you're preparing for an audit, expanding your facility, or seeking airflow system consultation, let VCR support you from initial survey to ISO/GMP-compliant reporting.
Contact VCR to get:
- Tailored airflow validation solutions for your factory scale
- Equipment and personnel recommendations
- Supply of certified tools: FFUs, pressure gauges, smoke kits
Hotline: 090.123.9008
Email: [email protected]
Website: https://cuaphongsachvcr.com/
Diep VCR














