Introduction of QMS - Quality Management System
A Quality Management System, commonly referred to as a QMS, comprises a set of internal guidelines established through a combination of policies, processes, documented procedures, and records.
What is QMS?
A Quality Management System, commonly referred to as a QMS, comprises a set of internal guidelines established through a combination of policies, processes, documented procedures, and records. This framework outlines how a company intends to achieve the production and delivery of its products and services to customers. When integrating a QMS into your organization, it should be tailored to suit the specific product or service offerings, emphasizing the importance of customization. However, to assist in ensuring comprehensive coverage, general principles are outlined in ISO 9001:2015 (Quality Management System – Requirements). These guidelines serve to standardize the design of a QMS.

Several QMS formats
Quality Management Systems (QMS) can be implemented in different formats to suit organizational needs and preferences, including electronic, paper-based, and hybrid solutions.
Here's an overview of the primary QMS formats:
eQMS
The Electronic Quality Management System (eQMS) is a digital solution aimed at enhancing and automating quality management processes within an organization.
Common variations of the term "Electronic Quality Management System (eQMS)" include:
-
eQMS
-
eQMS software
-
QMS software
-
Digital QMS
-
Quality management software
An eQMS is comprised of a suite of QMS tools, which are essentially software modules and predefined workflows created to automate and streamline specific quality management processes.
QMS software typically integrates various elements of quality management, such as document control, change control, training management, corrective and preventive actions (CAPA) management, audit management, supplier management, and more.
Paper-based
A paper-based Quality Management System (QMS) relies on physical documents for recording and tracking quality-related information. It offers simplicity and tangible documentation, making it potentially more cost-effective and accessible for smaller companies. However, for larger companies, the drawbacks of paper-based QMS can include higher costs in document control, limited scalability, and reduced efficiency.

Hybrid
The Hybrid QMS integrates elements of both paper-based and electronic systems to manage quality management processes effectively.
In this system, some quality management processes and documentation are maintained in traditional, physical formats, while others utilize electronic tools and technologies.
The Hybrid QMS allows companies to leverage the familiarity of paper-based systems while incorporating the benefits of digital tools.
This hybrid approach balances innovation with tradition, providing flexibility but also introducing potential inefficiencies and data inconsistencies in managing dual systems.
Types of QMS
A Quality Management System (QMS) can be based on either domestic or international standards, with various QMS options tailored to different needs and contexts. Organizations have the flexibility to adopt a single approach or blend different methodologies. Here are some of the most common:
-
Standardized systems: These systems establish benchmarks based on recognized standards and agreed-upon codes and practices, such as certifications against ISO standards. ISO 9001 delineates the requirements for a comprehensive QMS and offers guidance for organizations seeking to implement or enhance their quality management strategies.
-
Total Quality Management (TQM): TQM is a management philosophy centered on achieving customer satisfaction through the active involvement of all employees. Its objective is to continuously improve quality across all levels and functions of the business.
-
Lean Management: Inefficiencies often lead to unnecessary waste. Lean management aims to maximize customer value while minimizing waste by employing tools such as value stream mapping. This method helps refine an organization's processes for optimal efficiency.

-
Six Sigma: Although perfection may be elusive, the pursuit of excellence remains valuable. Six Sigma employs data-driven techniques to strive for near-perfect products and services, aiming for a defect rate of 3.4 per one million opportunities. While not absolute perfection, this is a remarkably close standard to achieve.
What is the purpose of QMS?
The objective of integrating a QMS is to maintain a consistent level of quality in products or services, particularly crucial as organizations expand, ensuring everyone consistently meets standards during manufacturing or service provision.
For many companies, especially in the life sciences sector, having a QMS is essential. However, even companies in diverse manufacturing and service industries can gain advantages from a QMS. Although establishing an efficient QMS may seem like a laborious task of paperwork and documentation, which can be both time-consuming and costly, effective implementation can lead to cost savings across the entire business.
Benefits of QMS
Quality Management Systems (QMS) offer companies a range of benefits, resulting in more efficient, cost-effective, and safe processes. Here are the primary advantages of implementing a QMS:
-
Improved Regulatory Compliance: QMS ensures companies adhere to industry regulations, standards, and guidelines, minimizing the risk of non-compliance and associated legal repercussions.
-
Enhanced Customer Retention and Satisfaction: Consistently delivering uniform and high-quality products or services through QMS boosts customer satisfaction, fosters loyalty, and increases repeat business.
-
Promotion of Continuous Improvement: QMS cultivates a culture of ongoing improvement, empowering companies to identify areas for enhancement, implement changes, and optimize processes over time.
-
Establishment of Operational Consistency: QMS sets standardized processes, leading to reliable and consistent outcomes, reducing variability, and ensuring a uniform approach across operations.
-
Improved Internal Communications: Effective communication within the company is promoted by QMS, ensuring all stakeholders are well-informed, and enhancing collaboration and teamwork.

-
Streamlined Employee Training: QMS provides a structured framework for employee training, ensuring staff members are adequately trained and qualified for efficient performance in their roles.
-
Increased Efficiency and Reduced Waste: QMS identifies and eliminates inefficiencies, resulting in streamlined processes, reduced waste, and optimized resource utilization, leading to cost savings.
-
Facilitated Data-Driven Decision-Making: QMS offers insights into processes and performance, enabling informed and strategic decision-making at all organizational levels.
-
Improved Company Culture: QMS fosters a culture of quality, accountability, and continuous improvement, creating a positive work environment and aligning employees with organizational objectives.
-
Enhanced Profits: Ultimately, QMS contributes to increased profitability through improved efficiency, customer satisfaction, and reduced costs, positioning companies for sustainable growth and financial success.
Why is a QMS important?
Every organization aspires to achieve excellence because ultimately, the quality of a product or service is what customers receive and are willing to pay for. Quality management plays a pivotal role in delivering a superior experience, which in turn significantly impacts a company’s growth and performance.
Here are six compelling reasons to consider investing in a quality management system (QMS):
-
Brand Reputation: A priceless asset, a brand is more likely to gain international recognition when an organization exceeds established quality benchmarks.
-
Customer Retention: Consistently meeting or surpassing customer needs and expectations fosters loyalty. When high standards are consistently upheld, customers are less likely to seek alternatives.
-
Business Sustainability: Ensuring excellence consistently guarantees and maintains a steady customer base. Operating sustainably, with minimal waste, is the optimal approach to growing and future-proofing an organization.

-
Compliance: Meeting regulatory, safety, and quality standards is essential, and a QMS seamlessly facilitates this process, ensuring compliance is maintained.
-
Competitive Edge: Higher-quality products and services provide businesses with a competitive advantage, especially in challenging market conditions.
-
Staff Engagement: Employees who feel involved in quality improvements tend to exhibit higher levels of engagement and productivity, contributing positively to the organization's success.
Requirements for QMS
Every component of a quality management system contributes to achieving the overarching objectives of meeting both customer and organizational requirements. While quality management systems should be tailored to address an organization's specific needs, they commonly include the following elements:
-
The organization’s quality policy and quality objectives
-
Procedures, instructions, and records
-
Data management
-
Internal processes
-
Customer satisfaction derived from product quality
-
Opportunities for improvement
-
Quality analysis
These elements form the foundation of a comprehensive quality management system, ensuring alignment with organizational goals and the delivery of high-quality products or services.
7 Quality Management Principles
The ISO 9001 Quality Management System is underpinned by seven critical principles, each essential for an effective QMS:
-
Customer focus: Understanding and meeting customer needs is paramount for product and service provision, forming the basis for enhanced customer satisfaction.
-
Leadership: Top management must provide resources and continually review the QMS for its success.

-
Engagement of people: Recognizing employees as a vital asset, their knowledge and experience must be integrated into the QMS.
-
Process approach: Every activity within an organization is a process, taking inputs and producing outputs, whether physical, informational, or energetic. Understanding these interactions forms the foundation of an effective system.
-
Improvement: Continuous improvement is essential for a company's survival, necessitating a QMS that constantly seeks better ways of operation.
-
Evidence-based decision making: Effective management requires an understanding of how processes are functioning. By basing decisions on factual data, the QMS can be managed and improved effectively.
-
Relationship management: Improving products and services often involves working with suppliers to enhance their offerings. Managing these relationships is crucial for overall improvement.
These principles are detailed in ISO 9000, a supplementary document supporting the ISO 9001 requirements.
Core elements of QMS
A Quality Management System (QMS) comprises essential core elements or modules that collaborate to guarantee a company’s products or services consistently meet regulatory and customer demands.
Tailored to the company and its industry, a QMS should reflect specific processes and requirements. While certain components are mandated by standards, regulations, and guidelines, others are optional.
Highlighted below are some core elements of a QMS:
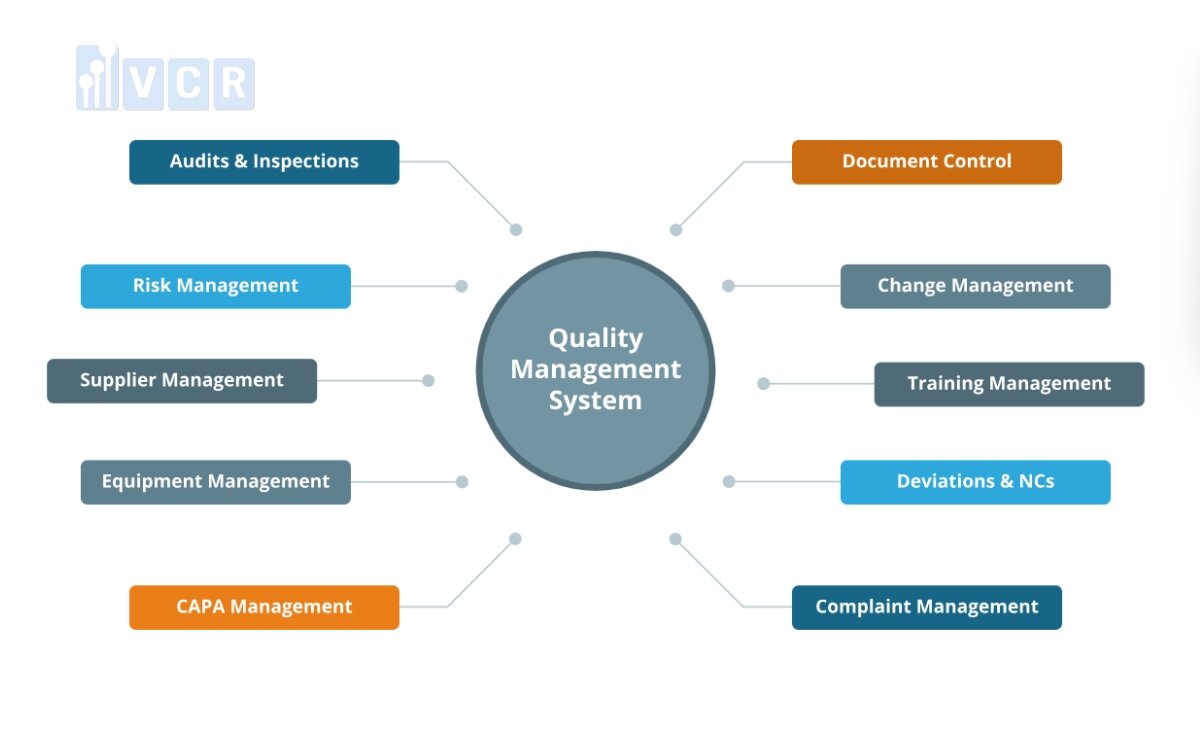
QMS document structure
The cornerstone of a Quality Management System (QMS) is its documentation, with the Quality Manual serving as the defining document for a company’s quality system. QMS documentation encompasses policies, procedures, work instructions, records, and other pertinent documents.
The structure of QMS documentation follows a hierarchical organization, facilitating understanding, communication, and visualization of the documentation's layout.
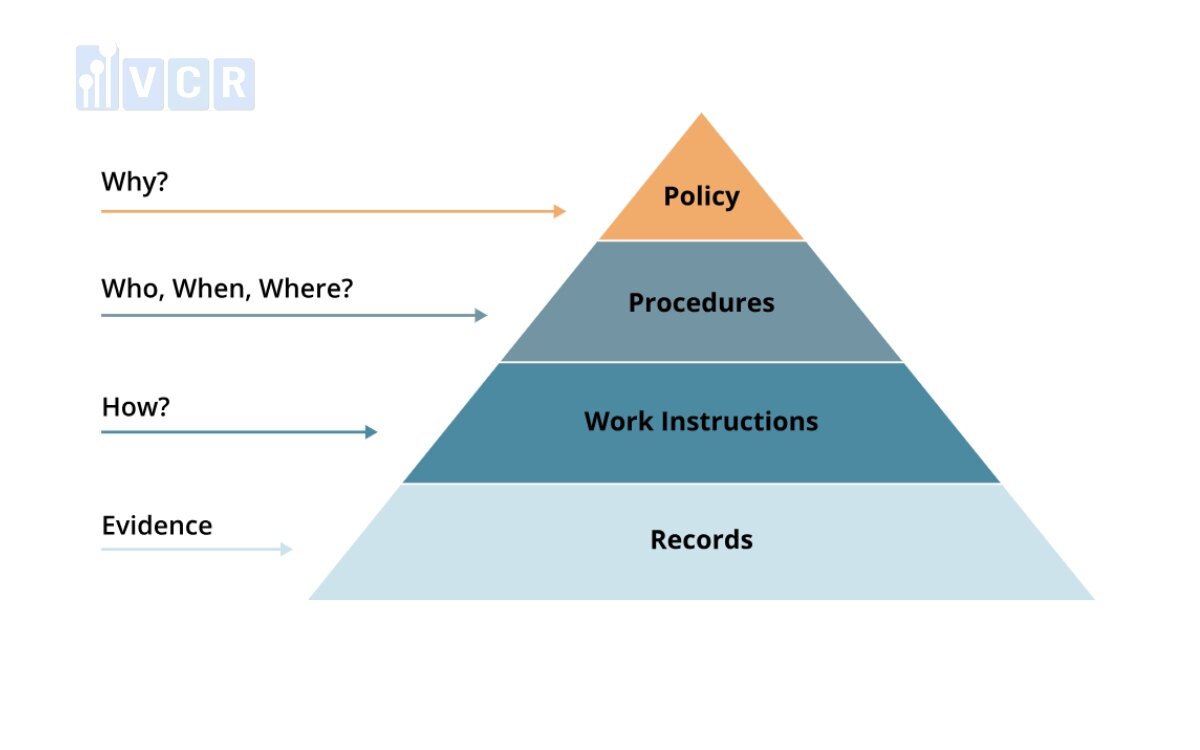
Here are the four levels of documents in the QMS pyramid:
-
Quality Policy: At the apex of the pyramid is the quality policy, a high-level statement articulating a company’s dedication to quality. It establishes the overarching direction for quality endeavors and acts as a guiding principle for employees.
-
Procedures: Next in the hierarchy are procedures, which delineate the step-by-step methods or processes for executing specific tasks or activities. Procedures aim to standardize operations, ensure consistency, and facilitate compliance with regulatory standards.
-
Work Instructions: Below procedures are work instructions, which offer detailed guidelines for completing specific tasks or operations, often at a more detailed level than procedures. Work instructions are essential for ensuring tasks are performed accurately, safely, and efficiently.
-
Records: The foundation of the pyramid consists of records, documenting the outcomes, activities, or events associated with quality management processes, such as audits and training. Records serve as evidence of performance and compliance, enabling traceability and accountability within the QMS.














