What is Quality Manual? ISO 9001 Quality manual
According to ISO 9001, the quality manual is a documented statement of an organization's quality management system.
Quality manual
What is it?
According to ISO 9001, the Quality manual is a documented statement of an organization's quality management system (QMS). It outlines the scope of the QMS and describes the system's procedures, processes, and resources needed to achieve quality objectives. The ISO 9001 standard does not prescribe a specific format or content for the quality manual, allowing organizations to tailor it to their specific needs and requirements. However, it typically includes sections such as the quality policy, objectives, organizational structure, responsibilities, and procedures for addressing key quality management processes like document control, corrective actions, and internal audits.
The quality manual serves as a reference document for demonstrating compliance with ISO 9001 requirements and communicating the organization's commitment to quality to stakeholders.

Who needs a Quality manual?
Users of the quality manual can include people inside and outside the company, related to the organization's production and business activities:
-
Leader
-
Department heads
-
Internal assessment expert
-
Evaluation and Certification organization
-
Agency
Watch more: 7 steps to building an effective quality control plan
The importance of Quality Manual?
Every organization should possess a quality manual, as it serves a crucial role. This document delineates the functioning and maintenance of your quality management system (QMS), acting as a permanent reference point.
By documenting the components of your QMS, you gain the ability to scrutinize and assess existing procedures, thereby identifying and rectifying inefficiencies. This proactive approach minimizes the likelihood of your QMS evolving haphazardly.
Additionally, a quality manual ensures that vital business information is not solely reliant on individual employees' memory. Dependence on any one employee for QMS knowledge poses a risk should they depart, potentially taking critical information with them. Moreover, a quality manual enhances communication within the organization, offering new employees insight into its objectives and clear vision from the outset. Furthermore, having a quality manual streamlines auditing processes, providing auditors with a structured document to review, thereby saving time and demonstrating organizational compliance.
Is Quality manual mandatory?
In the previous version of ISO 9001, quality manuals were compulsory documentation during audits.
The updated version of ISO 9001 in 2015 no longer requires this mandatory regulation, but imposes stricter requirements in the form of written information. This information needs to be verified to ensure the effectiveness of the quality management system.
Why do you need a Quality manual?
The quality manual is one of the important documents that businesses should have.

Because:
-
It records how the business manages and maintains the quality system, providing information for management.
-
It meets the requirements of the quality management system.
-
It reviews and analyzes, identifying existing weaknesses within the organization so that the business can then provide directions for improving and developing the system.
-
It documents all information about the quality system.
-
It makes it easier for new personnel to understand the goals, missions, and vision of the business.
-
It communicates management commitments to the quality system.
-
It saves time for assessors.
The difference between Quality manual and Quality plans
If you’re responsible for introducing a quality management system into your company or department, you must understand the differences between a quality manual and a quality plan. Though these documents go hand in hand with an organization’s quality management, they entail different things.

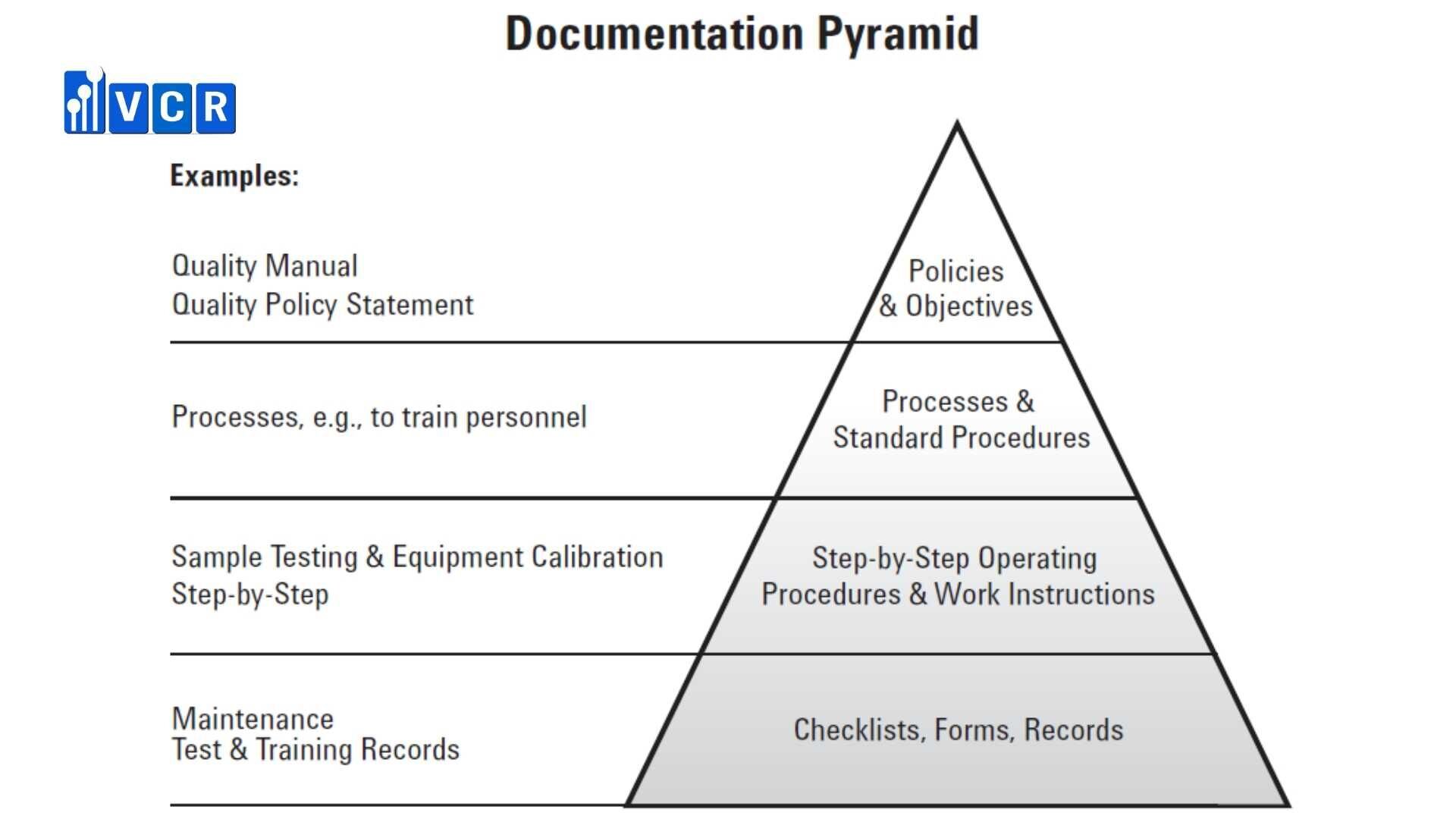
Quality Manuals
Quality management system manuals are sets of documents used to clearly define and communicate a company’s quality management system. These resources serve as a framework for meeting quality system requirements and convey a business’s commitment to this system.
QMS manuals comprise all the following records:
-
Quality policy statement: This document details the level of commitment an organization exhibits in maintaining quality.
-
Quality policies: The quality policy outlines a company’s plans in great detail and includes extensive documentation.
-
Standard operating procedures: These procedures identify the parties responsible for carrying out each activity and their allotted time frames.
-
Work instructions: Work instructions highlight the activities and procedures set to occur.

Quality Plans
Quality plans comprise a collection of documents specifying a quality management system’s quality standards, resources, specifications, practices, and all activity sequences relevant to a specific project, product, or service. Their purpose is to identify all organizational requirements that must be established to produce a product or service as intended.
Quality plans outline all procedures that fall under the following activity types:
-
Quality assurance: These activities include all proactive pre production efforts, such as developing quality standards and creating process checklists, and striving to improve process accuracy
-
Quality control: Quality control procedures refer to reactive post-production efforts focused on identifying any defective goods or services. Examples include product monitoring and inspections.
In short, a quality manual defines a business’s quality management system, while a quality plan explains how a company will meet quality requirements for a specific product or service.
Watch more: The 7 principles of quality management
Uses of Quality manual
Apart from its utility as a managerial instrument, the manual serves various other crucial functions:
-
Conveying management's expectations to employees.
-
Demonstrating the company's strategy for complying with the stipulations of ISO 9001:2015.

-
Showing compliance with Clause 5.3, which mandates the assignment, communication, and comprehension of organizational roles, responsibilities, and authorities.
-
Offering a reference for auditors, whether internal, customer-associated, or from the ISO certification body.
Quality manual contents
For ISO 9001:2015 users, the contents of your quality manual are entirely customizable, largely contingent upon your processes. ISO mandates the demonstration of evidence regarding the intentions, actions, and outcomes of your Quality Management System (QMS). When deciding how to structure your quality manual and what to incorporate, ensure that your primary focus is on aligning the included policies with your actual practices.
Some common topics typically included in quality manuals:
-
Quality policy
-
Description of the company’s documentation framework
-
Organizational structure chart
-
Policy statements addressing relevant ISO requirements
-
References to operational procedures
While these topics are not obligatory per ISO standards, your quality manual may cover some or all of them, or even more. The key lies in striking a balance between comprehensive coverage and essential inclusions that significantly impact the manual's effectiveness.
How do you prepare a Quality manual?
To ensure that your quality manual aligns with the requirements of your quality management system or ISO 9001, it should:

-
Clearly assign appropriate roles for each process.
-
Present information in a clear and concise manner.
-
Strike a balance between being detailed and avoiding unnecessary length.
-
Clearly delineate between each clause or section for ease of reference.
How to create a Quality Manual
Create an Outline
-
Define the purpose and scope of the manual and your organization.
-
Outline major policies and their importance.
-
Include comprehensive definitions applicable throughout the manual.
-
Provide insights into your company's history.
-
Mention a detailed organizational structure, management roles, and company responsibilities.
-
Describe facilities and relevant resources.
-
Outline methods, procedures, handling, storage, recordkeeping, accident prevention, safety, and corrective actions.
Prepare Section Details
-
Accurately collect and mention information for each section.
-
Validate procedural rules with relevant departments and governing organizations.

Present Steps Clearly
-
Express steps in an easy-to-understand language.
-
Define necessary terms and avoid jargon.
Create Clear Headings
-
Ensure consistency in presenting information.
-
Craft clear headings and subheadings for each section.
Add Table of Contents
-
Include a table of contents at the beginning once the manual is completed.
-
Optionally, add an index if relevant or required.
Review and Revise
-
Conduct a thorough review, checking for typographical errors.
-
Scrutinize policy descriptions for accuracy.
-
Ensure all information is precise to prevent corrective actions during audits.
FAQs about Quality manual
What is the primary objective of a quality manual?
The main goal of a quality manual is to effectively communicate information, demonstrate managerial commitment, and provide a framework for meeting the requirements of the quality system.

How often should a quality manual be updated?
Every organization should conduct an annual review of its quality manual.
How do ISO standards influence the content of a quality manual?
Adhering to ISO standards for quality manual content helps organizations establish operational frameworks and set performance expectations for their teams.
Who should participate in the development of a quality manual?
A steering committee should be actively involved in crafting the quality manual to ensure alignment with the specific needs of the organization.
Is it possible to have a fully digital quality manual?
Yes, a digital manual offers the most convenience and several advantages compared to traditional formats.














